

Pacific Grove, CA: Brooks/Cole, 2005.īrownian motion is the constant but irregular zigzag motion of small colloidal particles such as smoke, soot, dust, or pollen that can be seen quite clearly through a microscope. Englewood Cliffs, NJ: Prentice Hall, 2005. His results confirmed Einstein ’s analysis and put to rest any doubts about the molecular nature of matter. In an effort to verify Einstein ’s theoretical work, Jean Perrin carried out a number of experiments using small uniform particles of known size and mass.
Brownian motion series#
Early in the twentieth century, Albert Einstein (1879 –1955) published a series of papers in which he statistically analyzed the expected velocity of particles of various sizes and masses undergoing Brownian motion at various temperatures in liquids with different viscosities. The fact that the jiggling movement of a particle exhibiting Brownian motion increases with temperature provided evidence that its motion could be explained by the kinetic molecular theory. Your motion would be similar to that of a tiny pollen particle suspended in, and constantly being struck by randomly moving molecules of water.

A short time later, there might be more people pushing from behind than from in front, and you would move forward. At other times, more people would be bumping you from the right than from the left and so you would move to the left. Sometimes pushes from all directions would be equal and you would not move. You would find people bumping into you from all sides. Imagine yourself caught in the middle of a large crowd of people who are undecided as to which way they should go. When that happens, the particle will respond to the unbalanced force and move accordingly. However, since molecular motion is random, there will be moments when the particle is struck by more molecules moving in one direction than in any other. Since particles such as pollen are thousands of times larger than water molecules, we would expect that on the average the particle would be hit as many times by water molecules on one side as it would be by molecules striking it from the opposite direction. According to this theory, Brownian motion is the result of collisions between the small microscopic particles and the invisible but constantly moving water or air molecules surrounding them. Others found the same strange motion when they observed tiny inanimate particles of dye, dust, smoke, or soot.īrown could offer no explanation for his observation, which became known as Brownian motion nor could anyone else, until James Clerk Maxwell (1831 –1879) and others developed the kinetic molecular theory a generation later. He thought that the motion might be related to life processes within the pollen, but later he observed the same kind of zigzag motion with pollen from plants that had been dead for many years. As he watched the tiny particles of pollen under his microscope, Brown noticed that they were constantly jiggling about. In 1827, Robert Brown (1773 –1858), a Scottish botanist, prepared a slide by adding a drop of water to pollen grains.
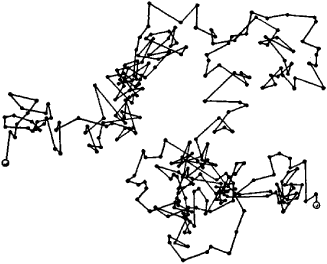
Image used with permisison (CC -BY-Sa 2.Brownian motion is the constant, but irregular, zigzag motion of small colloidal particles such as smoke, soot, dust, or pollen that can be seen quite clearly through a microscope.

This is a simulation of the Brownian motion of a big particle (dust particle) that collides with a large set of smaller particles (molecules of a gas) which move with different velocities in different random directions. The direction of the force of atomic bombardment is constantly changing, and at different times the particle is hit more on one side than another, leading to the seemingly random nature of the motion. Perrin was awarded the Nobel Prize in Physics in 1926 "for his work on the discontinuous structure of matter". This explanation of Brownian motion served as convincing evidence that atoms and molecules exist, and was further verified experimentally by Jean Perrin in 1908. Atoms and molecules had long been theorized as the constituents of matter, and Albert Einstein published a paper in 1905 that explained in precise detail how the motion that Brown had observed was a result of the pollen being moved by individual water molecules. In 1827, while looking through a microscope at particles trapped in cavities inside pollen grains in water, he noted that the particles moved through the water but he was not able to determine the mechanisms that caused this motion. This transport phenomenon is named after the botanist Robert Brown. \)īrownian motion is the random motion of particles suspended in a fluid (a liquid or a gas) resulting from their collision with the fast-moving atoms or molecules in the gas or liquid.


 0 kommentar(er)
0 kommentar(er)
